With a unifying theme of Microsystems Engineering for Life Sciences, the Microfluidics and BioMEMS Laboratory investigates the design and fabrication of novel microfluidic devices and micro/nano/soft robotic systems to address pressing problems in biology and medicine. Based on our interdisciplinary expertise in microdevices and robotics, our current research is along four strategic directions: i) paper-based microfluidic biosensing; ii) flexible/stretchable sensors and electronics; iii) microrobotic manipulation of cells and small organisms; and iv) soft robotics.
1. Paper-based microfluidic biosensing
Microfluidic devices made from patterned cellulose paper, commonly called “microfluidic paper-based analytical devices (μPADs)”, represent a low-cost yet powerful platform technology for biosensing applications. Since 2011, we have focused on the design, fabrication, and diagnostic applications of paper-based microfluidic biosensors. On μPAD design, we proposed new designs of paper-based timing valves [J27, J71 - see publication list] for on-chip fluid manipulation. We also explored the use of nanofibrillated cellulose (NFC)-based transparent paper (nanopaper) for μPAD fabrication and proposed a new concept of transparent nanopaper-based analytical device (nanoPAD) suitable for optical detection methods [J74, J80]. On device fabrication, we developed a new strategy for fabricating 3D microfluidic channels in a single layer of cellulose paper [J29], greatly simplifing the fabrication process of 3D-μPADs.
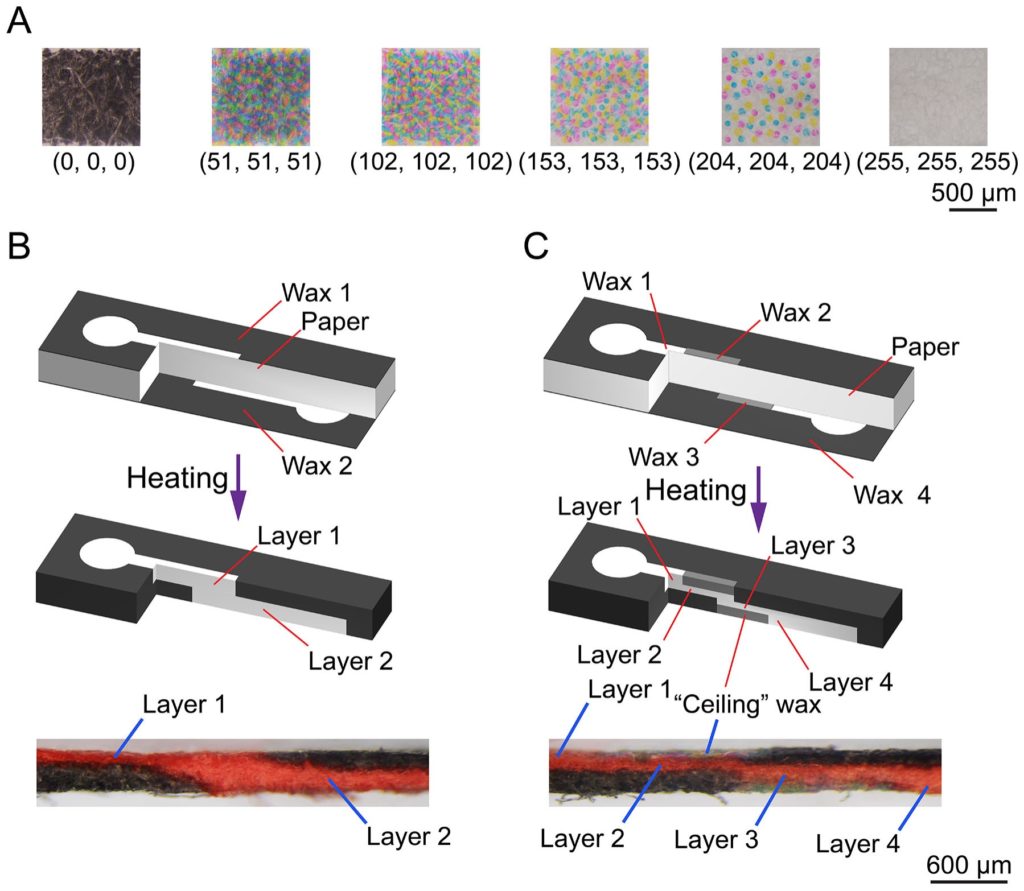
3D channel fabrication in a single paper layer [J29]
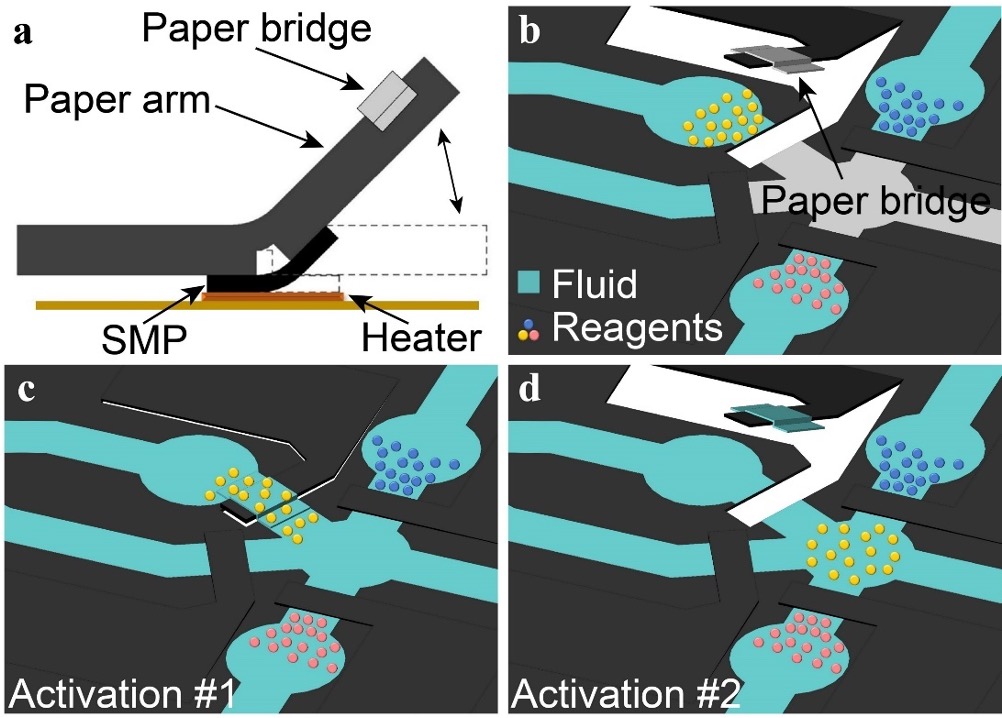
On-chip paper valve design [J71]
Targeting different life-threatening diseases, we have developed several paper-based diagnostic platforms and fully tested their clinical performance using patient samples [J28, J38, J42, J43, J71, J85]. We have filed five PCT/US patents and are working with industrial companies to commercialize those diagnostic platforms. Our research was regularly highlighted as journal covers and editor's picks [J38, J42, J43, J74, J80], and was widely reported by national and international media organizations. Most notably, our paper-based electrochemical biosensor [J43] for testing HIV and hepatitis C virus (HCV) co-infection was highlighted by the World Health Organization (WHO) as a next-generation technology for HIV/HCV diagnostics; our journal article [J38] published on Microsystems & Nanoengineering (IF: 7.1) received the 2020 Highly Cited Article Award.
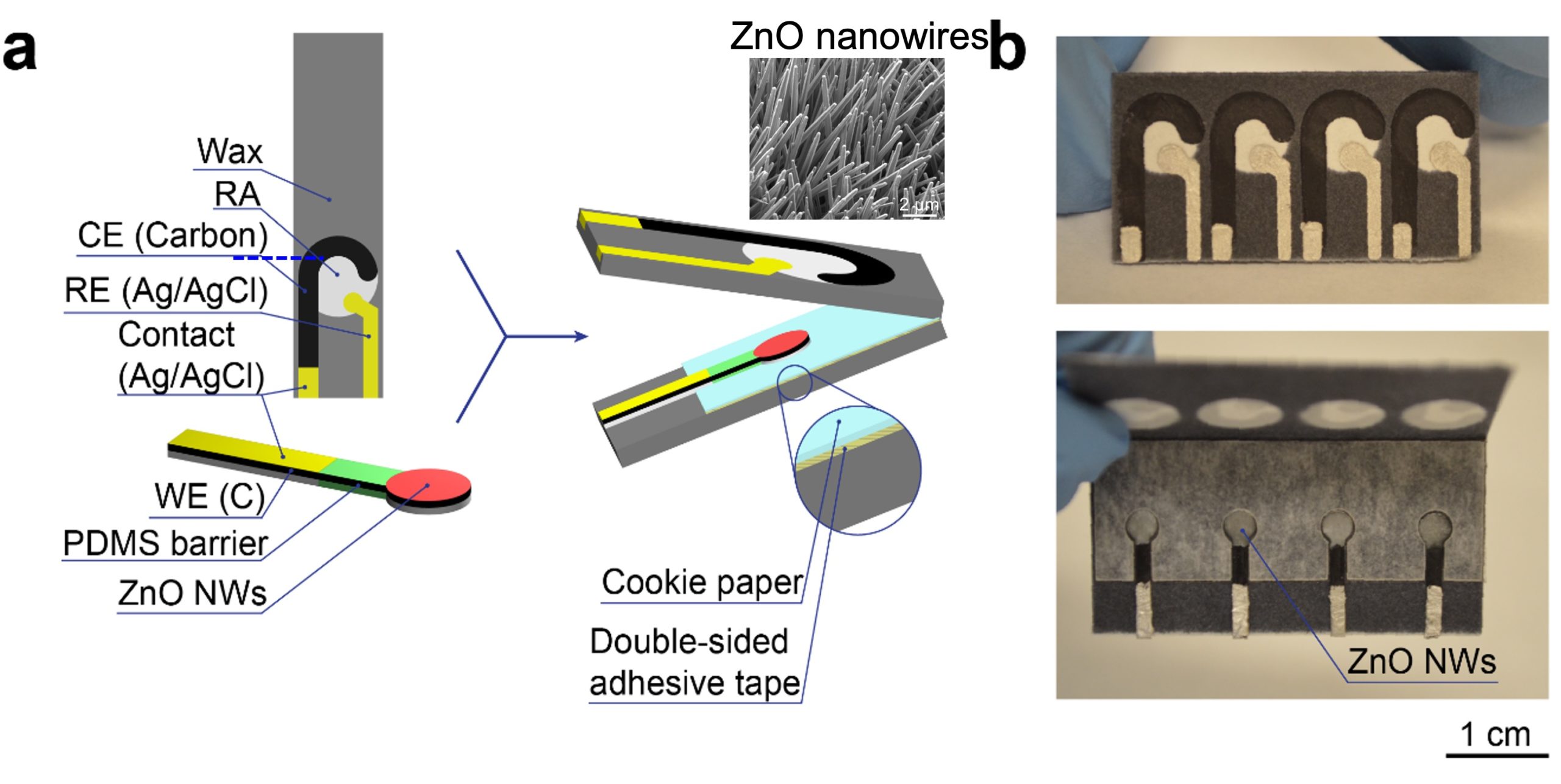
Paper-based electrochemical nanobiosensor for HIV diagnosis [J42]
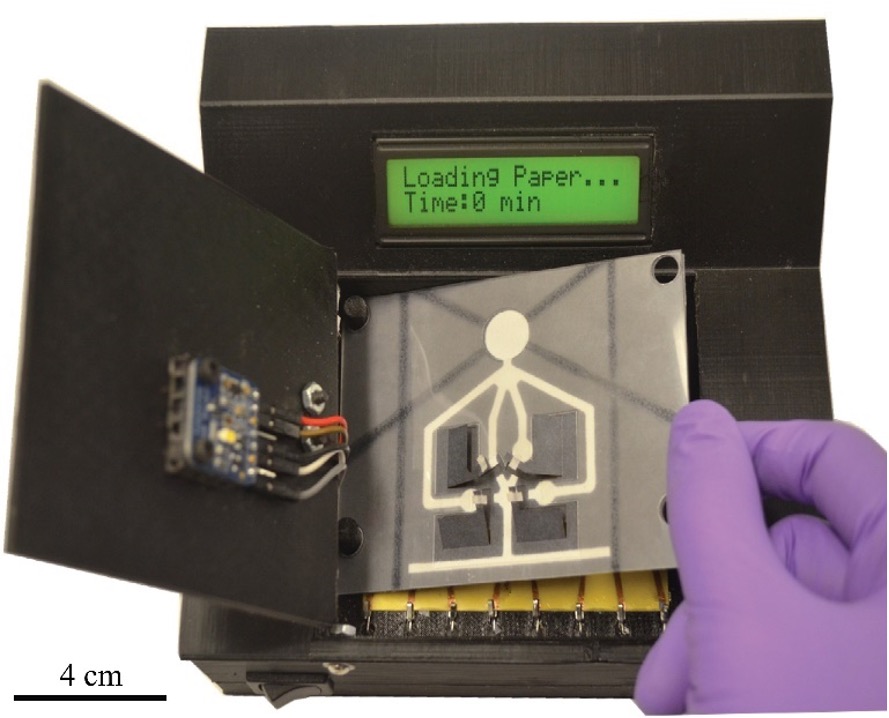
Automated ELISA platform for laryngopharyngeal reflux testing [J71]
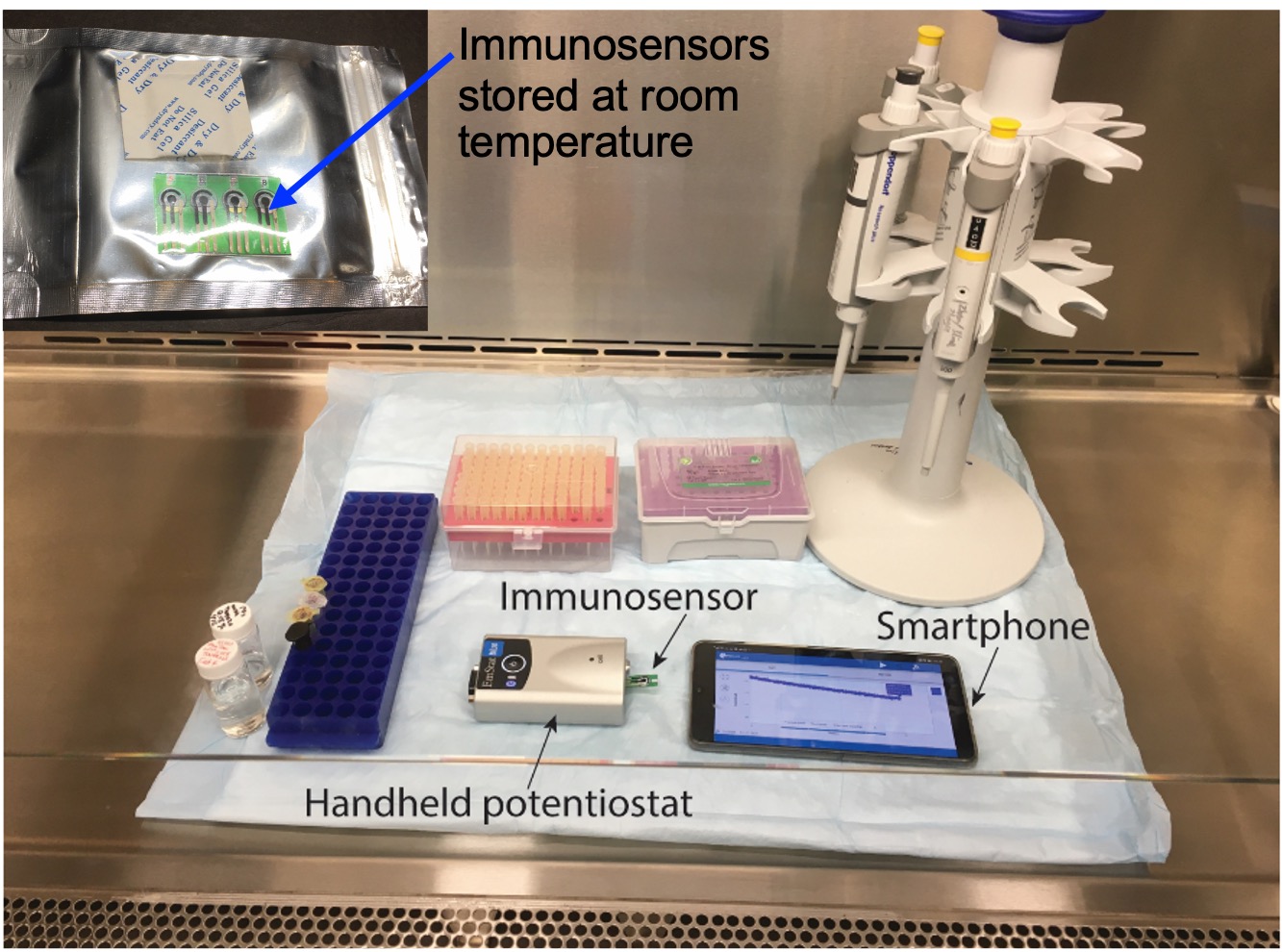
Electrochemical immunosensors for COVID-19 antigen and antibody testing [J98]
2. Flexible/stretchable sensors and electronics
Paper-Based Flexible Sensors: We pioneered the use of regular paper substrates for creating the first paper-based force sensor [J24]. We performed a systematic study on hydrothermal growth of zinc oxide nanowires (ZnO NWs) on regular paper substrates [J37]. Based on the ZnO-NW paper, we developed the first paper-based piezoelectric touch sensor for paper-based electronics [J33] and the first paper-based accelerometer for smart packaging [J57].Hydrogel-Based Ionic Skins: Our team recently developed a novel double-network hydrogel-based ionic skin (iSkin) capable of recapitulating the salient features of biological skin and tolerate extremely low temperature. We applied the iSkin to strain sensing on clothes, human skin, and soft robots [J92]. Based on a similar hydrogel material system, we also designed the first stretchable ionic diode with a bilayer diode junction. The unique property of the diode enabled the fabrication of a self-powered artificial ionic skin (AIskin) with strain and humidity sensing capability [J75]. This work won the 2020 Outstanding Article Award (1/355) from Materials Horizons (IF: 13.3).
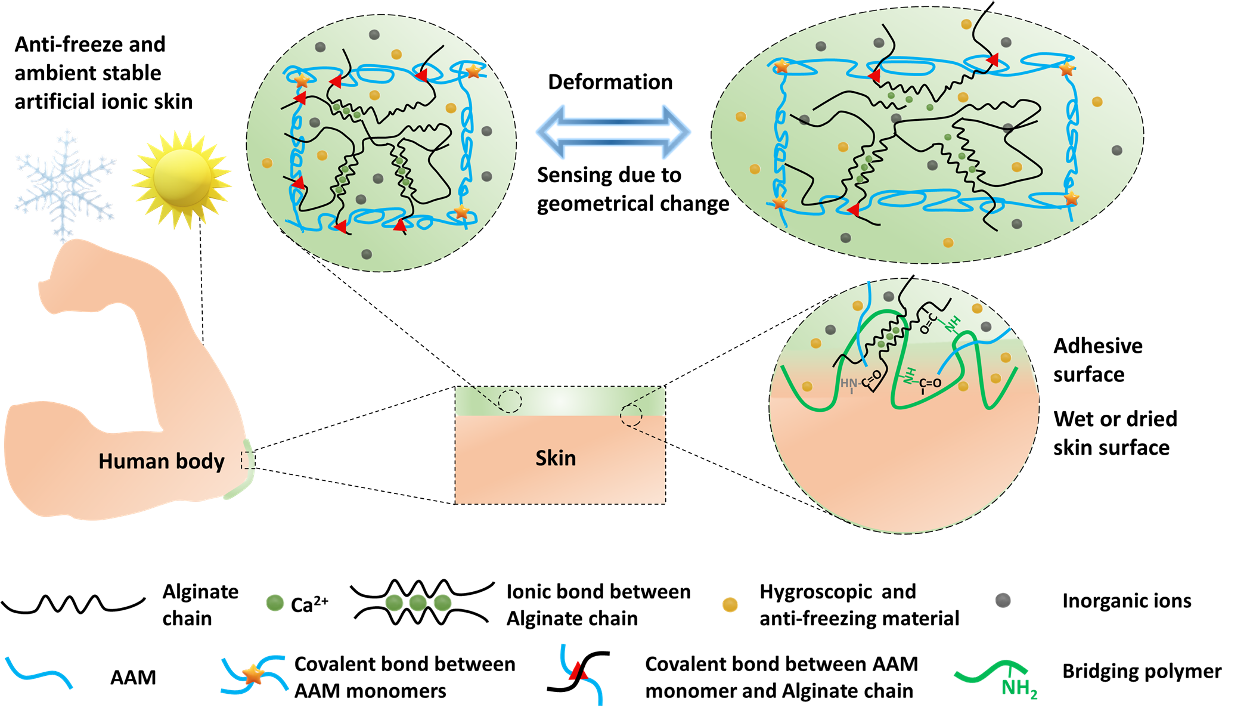

Bioinspired design of the hydrogel-based ionic skin (iSkin) [J92]
iSkin as a wearable strain sensor

iSkin glove for robotic hand control
Soft walking robot wearing iSkin sensors
3. Microrobotic manipulation of cells and small organisms
The capability of manipulating biological samples such as cells and small organisms is highly desired in biological and medical research. We developed the first automated microrobotic system for high-speed microinjection of zebrafish and mouse embryos [J10][J23]. Our team developed the first automated microrobotic system for high-speed injection of C. elegans [J41][J77] and are now using this high-throughput tool for large-scale gene editing on C. elegans. We have also developed the first force-controlled microrobotic system for mechanical stimulation and calcium fluorescence imaging on single Drosophila larvae [J39, J45, J50]. This system has proven highly efficient for studying neural mechanisms of the animal’s danger-escaping behavior [J86].
Robotic system for automated worm injection [J77]
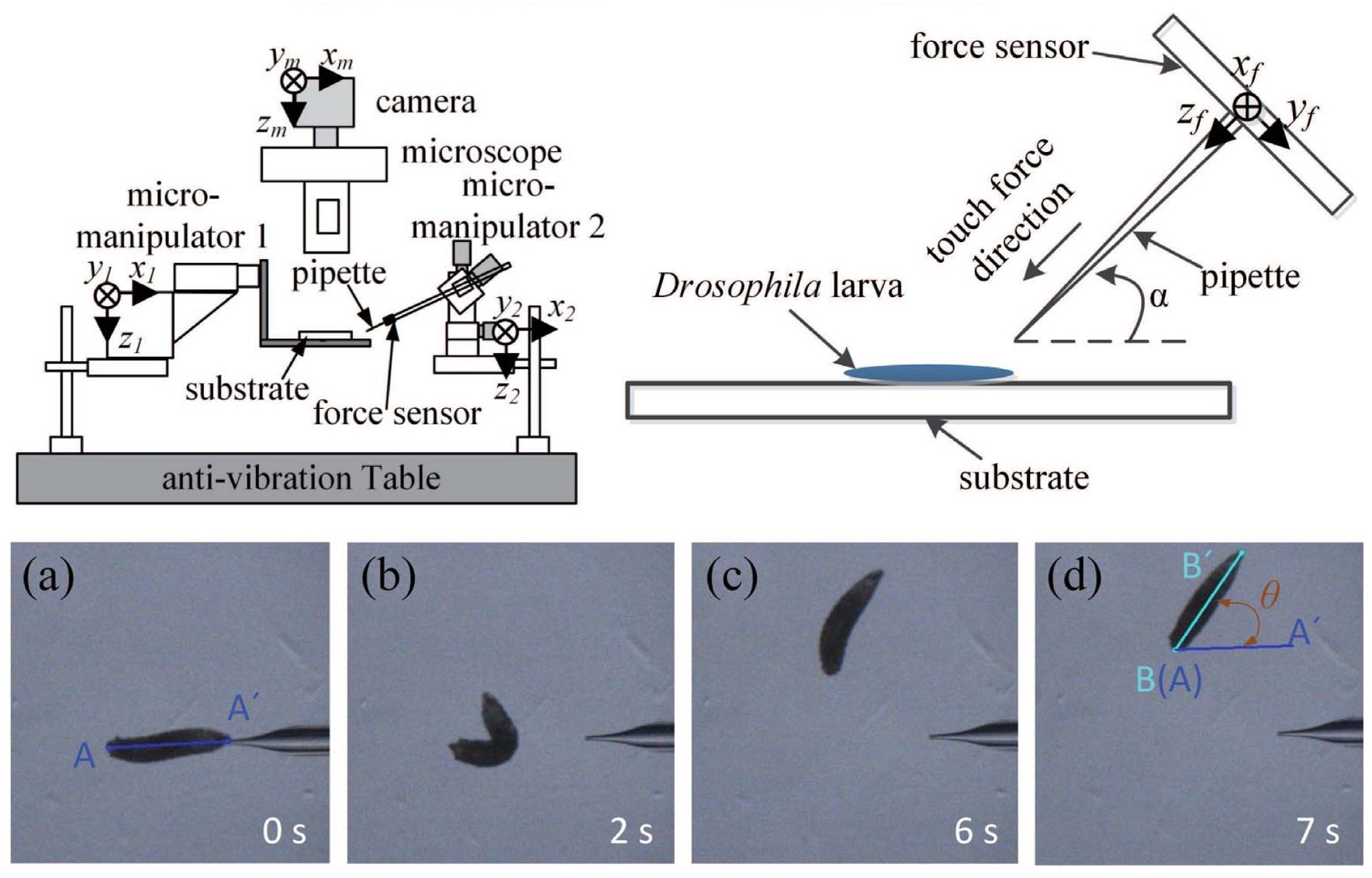
Force-controlled robotic system for Drosophila larva stimulation [J62]
4. RoboWorm: a living soft microrobotics
Leverage our unique expertise in C. elegans optogenetics and microrobotics, we recently developed an optically controlled soft-bodied microrobot directly from a live C. elegans (which is named “RoboWorm”) [J93]. Through optogenetic and biochemical methods, we shut down the worm’s signal transmissions between its neuronal and muscular systems but still maintain its muscle cells optically excitable. We developed a high-performance patterned-light illumination system to selectively target the worm’s body wall muscle cells and thus control its crawling. The RoboWorm can realize all normal worm crawling behaviors, and its the crawling direction and destination can be regulated through vision-based feedback control. This integrated approach infuses a machine mind into a biological body, enlightening a new paradigm of developing living soft microrobots.
Robotic locomotion control of a living C. elegans worm [J93]

